Abstract
Introduction: Chimeric antigen receptor (CAR) T-cell therapy has changed the treatment landscape of relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). However, there are still limited real-world evidence of survival outcomes from CAR T-cell therapy in older patients with DLBCL. The aim of the study was to examine the outcomes and economic impact among older patients with DLBCL receiving CAR T-cell therapy in a real-world setting in the United States.
Methods: Using administrative medical and pharmacy claims from the 100% Medicare Fee-for-Service database, older patients (≥65) with at least one inpatient or two outpatient claims with a diagnosis of DLBCL between 4/1/2016 and 12/1/2020, followed by at least one claim for the administration of CAR T-cell therapy between 1/1/2018 and 12/31/2020 were selected. Patients with evidence of clinical trial participation were excluded. Bridging therapies were reported based on the presence of therapy in the 4-week period preceding the infusion. Outcomes included progression-free survival (PFS) and overall survival (OS). The initiation of any subsequent treatment was used as a proxy for progression. Additionally, healthcare resource utilization (HrU) and total costs within the 90-day period following the infusion were examined among patients with ≥90 days of enrollment post-infusion. The proportion of patients receiving inpatient care, emergency room (ER) services, and outpatient services post CAR T-cell therapy, were reported by location of CAR T-cell therapy administration (inpatient vs. outpatient). Descriptive analyses included means, medians, and standard deviations (SD) for continuous variables, and frequencies and proportions for categorical variables. PFS and OS were analyzed using Kaplan-Meier curves, calculated from infusion date, and tested for significance using the Log-Rank test. Select analyses were further stratified by age category (65-69; 70-74; 75+ years).
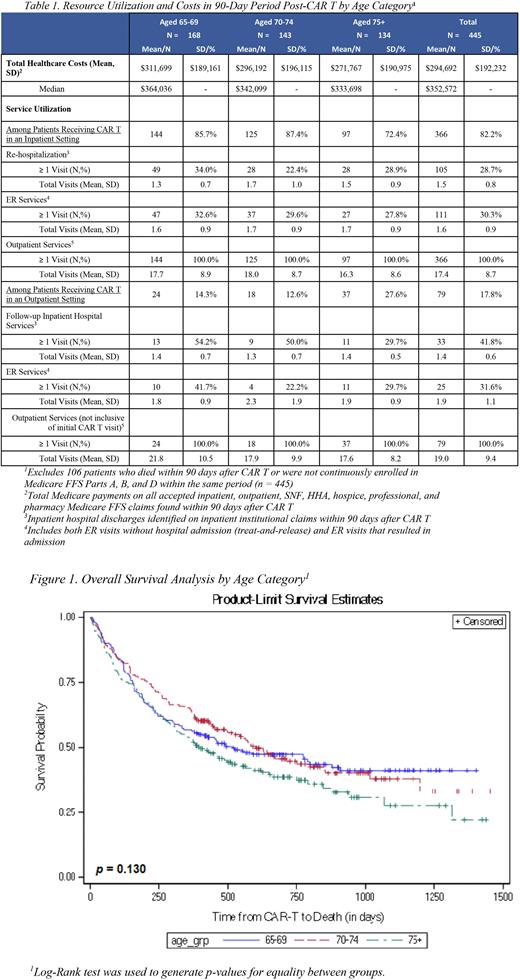
Results: A total of 551 patients with R/R DLBCL receiving CAR T-cell therapy were included, with mean age of 72.2 and 173 patients (31.4%) aged ≥ 75. A total of 262 patients (47.5%) received bridging therapy (Table 1). The oldest patients (≥75) had shorter median PFS (160 days) and OS (403 days), compared to patients aged 70-74 who had median PFS (379 days) and OS (603 days) and patients aged 65-69 who had a median PFS (194 days) and OS (518 days) (Figure 1). The 12-month failure rate based on PFS was highest in patients aged ≥ 75 at 66%, compared to patients aged 70-74 at 48% and patients aged 65-69 at 57% (p = 0.001). The majority of patients received CAR T-cell infusion in an inpatient setting (n=456, 82.8%), with an average length of stay 21.4 days. Within 90 days following infusion, 28.7% of patients who received CAR T-cell therapy in an inpatient setting presented a re-hospitalization, 30.3% had an ER visit, and all patients had ≥1 outpatient visit, with a mean of 17.4 outpatient visits. Among patients who received CAR T-cell therapy in an outpatient setting, 41.8% were hospitalized later, 31.6% had an ER visit, and all patients had ≥1 outpatient visit, with a mean of 19.0 outpatient visits. Similar HrU rates were observed across age groups, except hospitalization rate, which was lowest among patients with age ≥ 75. Total median cost within 90 days from the infusion date (inclusive of CAR T-cell infusion) was $352,572 and was similar across all age groups (Table 1).
Conclusion: This study is one of the largest real-world studies to date of patients with R/R DLBCL receiving CAR T-cell therapy leveraging Medicare claims data. Older patients in this study appeared to have shorter PFS and OS compared to clinical trials, with the worst outcomes observed in patients aged 75+. Similar costs within the 90-day period post CAR T-cell therapy were observed across age groups, and intensive use of HrU was noted. The results provide important reference to consider real-world healthcare utilization and cost effective treatment approaches in older patients with R/R DLBCL.
Disclosures
Chihara:Eisai: Honoraria; AstraZeneca: Honoraria. Liao:ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Tkacz:ADC Therapeutics: Consultancy. Lewing:Inovalon: Current Employment. Kilgore:ADC Therapeutics SA: Research Funding. Nastoupil:Genentech/Roche, MEI, Takeda: Other: DSMC; ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding. Chen:ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.